The 3rd International Nursing and Health Sciences Students and Health Care Professionals Conference (INHSP)
More infoThis study aims to identify scientific evidence and knowledge currently available related to clinical laboratory management in the face of disasters with the literature review method.
MethodThis is a literature review study. Various references were collected from online databases, including reports, scientific journals, and books in the last ten years. Articles are screened according to the research objectives. The keywords used are laboratories, hospitals, disasters, Point of Care Testing (POCT).
ResultsPOCT is defined as the tools of a laboratory diagnostic test used in a disaster situation effectively. It can be operated by anyone who has been trained. However, it is quite constrained by temperature, earthquake shocks, and battery life. Storage and reagents supply, such as the strip test, might be prepare before the disaster. Moreover, maintenance and validation of POCT tools in disaster preparation is necessary. Hospitals must prepare POCT to conquer the limitation of electricity sources, human resources, and buildings situation was using a laboratory diagnostic analyzer not possible. The POCT was suitable to be conveyed anywhere both within the hospital and to disaster sites using the vehicle.
ConclusionPOCT has the enormous potential as an emergency laboratory examination tool both at the hospital and at the disaster site to grip patient safety.
Natural disasters such as earthquakes, tsunamis, fires, landslides, liquefaction cause health crises, including paralysis of health services, deaths, injuries, refugees, nutritional problems, problems with the availability of clean water, environmental sanitation problems, infectious diseases, and psychological problems.1 Problems faced in handling health crisis due to disaster include information system that has not been running well, coordination mechanism that has not been functioning properly, mobilization of assistance from outside the disaster location is still hampered due to transportation problems, the financing system is not yet supported, the early awareness system is not yet going well and logistical limitations.2
In a disaster situation, the Hospital (RS) will be the final destination in dealing with victims so that the hospital must make adequate preparations. Preparation can be realized, among others, in the form of planning plans to deal with emergency situations or contingency plans, which are also intended so that the hospital can still function for patients who already exist (business continuity plan). The plan is generally referred to as the Disaster Management Plan at the hospital, or Hospital Disaster Plan (HDP).3 One important sector in disaster management in hospitals is the ability of clinical laboratories to remain operational and recover quickly after a disaster. The three main work areas of clinical laboratories in disaster or emergency conditions are diagnostic confirmation of infectious diseases with high mortality rates, the availability of standard diagnostic tests to treat injured victims, and the preparation and administration of blood products safely.4
In a disaster situation, the laboratory's response to emergencies will face many obstacles, including the difficulty of reaching the disaster site, lack of reagents, equipment, electrical power, and material suppliers, and insufficient staff. This condition may be exacerbated by the absence of supervision, access, training.4 The response of health services to disasters will improve if mitigation activities and the ability to secure, restore the functions of laboratory services are included in the emergency management plan. The Joint Commission stated that the laboratory must have its own emergency preparedness management even though the laboratory emergency response was included in the HDP.
Laboratory emergency preparedness management must be able to identify laboratory capabilities and provide immediate response 96h after a disaster occurs. The College of American Pathologists (CAP) mandates that laboratories must have internal and external policies and procedures in disaster preparedness.5 Appropriate disaster preparedness based on each stage of the disaster is expected to improve the ability of laboratory resources and facilities to provide optimal services in the midst of chaos. This study aims to identify scientific evidence and knowledge currently available related to clinical laboratory management in the face of disasters with the literature review method.
MethodThe main source of literature review in this study was a database in the network (online database) that provides journal articles in PDF formats, such as Pubmed, Proquest, Google scholar, and EBSCO. Other sources were textbooks from libraries, national guidelines, national data reports, theses, and dissertations. There were no limitations on the date of publication of the literature used as long as the literature found is relevant to the purpose of the study. However, to ensure that the information used is the most up-to-date literature, the information used uses more literature that has been published over the past ten years.
Results and discussionHospital clinical laboratory preparation during disaster and post-disasterSeveral studies have reported laboratory experiences in dealing with natural and human-made disasters. Many hospitals and laboratories suffered heavy damage, so the first step taken was to restore and replenish the destroyed laboratory services.6 One of the problems faced is the unavailability of shelters for storing laboratory equipment in an uncontrolled work environment. Changes in temperature drastically reduce the efficiency of using tools that are sensitive to changes in temperature and electricity supply in areas that have been severely damaged. The availability of generators is very limited, and if there are power fluctuations making it difficult to control the quality of laboratory results.7 Pasokan Regular supply and storage of sensitive laboratory reagents and diagnostic equipment are major challenges and clean water crisis to avoid water contamination.7
Therefore a strong laboratory management method and mechanism that includes integrated medical leadership and administration across all laboratory service lines enable the provision of appropriate and fast responses in case of disaster.8
Points of Care Testing (POCT) as one of the hospital's clinical laboratory efforts to overcome resource limitations when a disaster occursThe history of the use of POCT, which began in 1960, is that clinical laboratory examinations are practical and automatic so that technological development is smaller, their use is easy, especially for laboratory examinations in emergency departments. This was answered by making portable blood sugar (portable blood glucose). In the mid-1980s, it was introduced the examination technology to ensure accuracy (POCT), which at that time was in the form of the dipstick (dipstick) for the examination of urine (urine) quickly.
Point of Care Testing (POCT) is defined as an examination of diagnostic tests and laboratory tests carried out by officers with an educational background, not clinical laboratories, or conducted or done by the sufferer himself.9 POCT can be in the form of side tests (ancillary testing), satellite testing (satellite testing), bedside testing (bedside testing), near-patient testing (near-patient testing), home testing (home testing), outside laboratory testing (out of laboratory testing).10
The advantage of using POCT is that the results of the rapid examination are beneficial for doctors who treat patients, so they can analyze the development of the patient's condition, can take further treatment steps, and can discuss it with the patient or his family. It does not require handling samples, such as centrifugation. The use of POCTs does not need to use special staff who are educated in laboratory science but can be done by other health workers.11
The disadvantage of using POCT is due to easy and fast checks can lead to checks that are more than necessary or incorrect. The use of a small blood sample is difficult to know the quality of the sample that can affect the accuracy of the results of examinations with POCT, such as hemolysis, lipemia, and drugs. The use of POCT conducted by non-laboratory officers, requires quality management so that the results of the inspection are guaranteed and the arrangements in using it, so it needs to be regulated and determined who meets the requirements as POCT users.12
Some tests that can be done with POCT are blood glucose examination, hemoglobin A1c (HbA1c), blood gas analysis (AGD), cholesterol, hematological examination, and others.13 The laboratory examiner uses the POCT conducted by nurses or other health workers who do not have a basic education in laboratory science and laboratory technology. Thus, the feasibility of a POCT tool, which also involves accuracy (accuracy) and accuracy (precision) of the examination results, is the responsibility of the central laboratory. POCT quality assurance is recommended to be formally implemented as a support for risk management and reduce medical errors. Thus, management of quality management should be carried out by the parent laboratory, and the parent laboratory needs to set a gold standard.9,14
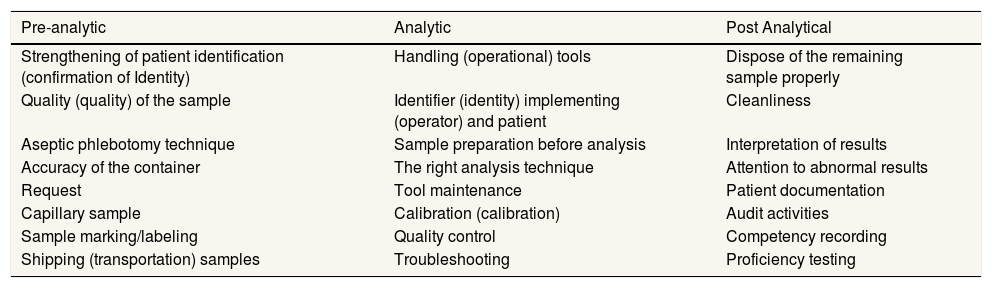
Quality assurance activities include the process of improving quality and strengthening quality. Quality improvement activities include strengthening internal and external quality. Quality assurance activities more broadly include activities to improve performance which include: pre-analytic, analytic, and post-analytic (Table 1).9
Process of POCT quality assurance activities.
| Pre-analytic | Analytic | Post Analytical |
|---|---|---|
| Strengthening of patient identification (confirmation of Identity) | Handling (operational) tools | Dispose of the remaining sample properly |
| Quality (quality) of the sample | Identifier (identity) implementing (operator) and patient | Cleanliness |
| Aseptic phlebotomy technique | Sample preparation before analysis | Interpretation of results |
| Accuracy of the container | The right analysis technique | Attention to abnormal results |
| Request | Tool maintenance | Patient documentation |
| Capillary sample | Calibration (calibration) | Audit activities |
| Sample marking/labeling | Quality control | Competency recording |
| Shipping (transportation) samples | Troubleshooting | Proficiency testing |
In this case, by consulting with POCT users or other health professionals, conducting periodic audits, periodically assessing service patterns with POCT, and evaluating the implementation of the quality assurance program by the quality assurance team. Research shows the use of POCT as one of the laboratory testing tools in conditions of limited resources due to disaster, namely POCT being an invaluable clinical tool in critical situations.15 However, the use of POCT is quite constrained by humidity, temperature, salinity, altitude, earthquake shock, and battery life while working at the rescue site. Storage and supply of reagents, test strips must be considered before a disaster occurs. It is necessary to improve the durability and validation of POCT tools in disaster preparation.16
The use of innovative POCTs, along with specific tactics, will increase disaster preparedness. Quality control and educational programs using POCT are required. Officers who work on POCs must be given information about the use of monitoring in cases of poor peripheral circulation and other confounding factors that can affect calibration or interfere with measurement results. Providing education to the POC coordinator will facilitate periodic assessments and adjustments to tactics, instrument innovation, and cost-effectiveness. The main test priorities according to the sequential response are complete blood count, electrolyte chemistry, blood type, oxygen saturation (using pulse oximeter), hematocrit, and microbiology as top priorities, and choosing to draw blood directly using cassettes. Cardiac biomarkers are needed at alternative service facilities. The main infectious diseases are Staphylococcus aureus, SARS, Streptococcus pneumonia, and hepatitis B. Temperature, vibration, humidity and the influence of vibrations are four important things that should be a concern in extreme situations.
ConclusionThe hospital will be the main destination for survivors when disaster strikes. When disasters occur, chaos will occur that can cause services to be suboptimal, coupled with limited resources both in the supply of equipment, materials, electricity, water, and health workers. Hospitals need to have a good hospital disaster plan to minimize chaos when disasters occur. Hospitals must also be prepared to deal with health problems that arise in post-disaster refugees.
Clinical laboratories in hospitals have an important role in disaster conditions. Laboratories must have pre-disaster and post-disaster emergency preparations. POCT has enormous potential as an emergency laboratory examination tool both in hospitals and at disaster sites to maintain patient safety.
Conflict of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the 3rd International Nursing, Health Science Students & Health Care Professionals Conference. Full-text and the content of it is under responsibility of authors of the article.