Edited by: Carme Borell
Last update: March 2023
More infoThe aim of this study was to analyze socioeconomic position (SEP) inequalities in the prevalence and incidence of type 2 diabetes mellitus (T2DM) in people aged 50 years and over in Europe and to describe the contribution of body mass index (BMI) and other possible mediators.
MethodsThis was a cross-sectional and longitudinal study including men and women ≥50 years old in 11 European countries in 2004 and 2006 (n=21,323). The prevalence and cumulative incidence of T2DM were calculated with self-reported T2DM or when the individual took drugs for diabetes. Prevalence ratio (PR) and relative risk (RR) of prevalent and incident T2DM were calculated according to educational level and adjusted by BMI and other possible mediators.
ResultsThe age-adjusted and country-adjusted prevalence of T2DM in 2004 was 10.2% in men and 8.5% in women. Compared to those with higher education, men and women with lower education had a PR [95% CI] of T2DM of 1.29 [1.12–1.50] and 1.61 [1.39–1.86], respectively. SEP-related inequalities in incidence (RR [95%CI]) were 1.88 [1.35–2.62] in women and 1.04 [0.78–1.40] in men. Adjusting for potential mediators reduced inequalities in the prevalence and incidence of T2DM among women by 26.2% and 21.6%, respectively, and inequalities in prevalence among men by 44.8%.
ConclusionsWe observed significant inequalities in the prevalence and incidence (women only) of T2DM as a function of socioeconomic position. These inequalities were mediated by BMI.
Analizar las desigualdades por posición socioeconómica en la prevalencia y la incidencia de diabetes mellitus de tipo 2 (DM2) en las personas de 50 o más años de edad en Europa, y describir la contribución del índice de masa corporal (IMC) y la de otros posibles mediadores.
MétodosEstudio de diseño transversal y longitudinal que incluye personas de 50 o más años de edad de 11 países de Europa, entre 2004 y 2006 (n=21.323). Se calculó la prevalencia y la incidencia acumulada de DM2 a partir del autorreporte de DM2 o de si la persona consumía fármacos para la diabetes. Se estimaron riesgos relativos (RR) de incidencia y razones de prevalencia (RP) de DM2 según el nivel educativo, y se ajustaron por el IMC y otros posibles mediadores.
ResultadosLa prevalencia de DM2 ajustada por edad y país en 2004 era del 10,2% en los hombres y del 8,5% en las mujeres. Comparado con las personas con un nivel de estudios más alto, los hombres y las mujeres con menor nivel de estudios tenían una RP (IC95%) de DM2 de 1,29 (1,12–1,50) y 1,61 (1,39–1,86), respectivamente. Las desigualdades por posición socioeconómica en la incidencia (RR [IC95%]) eran de 1,88 (1,35–2,62) en las mujeres y de 1,04 (0,78–1,40) en los hombres. Ajustando por los potenciales mediadores, las desigualdades en la prevalencia y la incidencia de DM2 se redujeron en las mujeres un 26,2% y un 21,6%, respectivamente, y las desigualdades en la prevalencia en los hombres un 44,8%.
ConclusionesSe observan desigualdades en la prevalencia y la incidencia (sólo en las mujeres) de DM2 en función de la posición socioeconómica. Estas desigualdades estaban mediadas por el IMC.
Diabetes is a growing global health problem, and an estimated 4.4% of the global population will have diabetes by 2030.1 The increased prevalence observed in recent years is mainly attributed not only to an increase in the incidence in type 2 diabetes mellitus (T2DM), but it could also be due to declining mortality among T2DM patients2–4 in some countries. This increased incidence is likely due to the increased prevalence of its main risk factors, such as obesity and sedentarism,1–6 while declining mortality among T2DM patients may be explained by improvements in treatment.3,4 Moreover, disease monitoring and improved access to health services play an important role in preventing diabetes-related complications.7
Inequalities in the incidence, prevalence and mortality of T2DM as a function of socioeconomic position (SEP) have been described in some populations.7–12 People with disadvantaged SEP (low income, deprived occupational social class and/or low educational level) experience higher rates of incidence and prevalence and are more likely to die from T2DM than those with high SEP.7 Some studies have suggested that the presence of SEP-related inequalities in T2DM are mediated13 by the unequal distribution of risk factors for T2DM between levels of SEP,9,14 and indeed obesity, physical inactivity, and unhealthy diet are most prevalent among those with the lowest SEP.15 For example, obesity has been reported to account for 26% and 36% of the variance in the prevalence of T2DM in middle-aged European men and women, respectively.16 However, there has been some debate regarding the existence of residual SEP-related inequalities in the incidence and prevalence of T2DM after adjusting for these traditional risk factors.9–12,14 It is also necessary to consider that these factors may in part be the result of the conditions in which people are born, grow, live, work and age, conditions that are shaped by the distribution of money, power and resources at global, national and local levels.
To date, no Europe-wide follow-up studies have been reported that analyze social inequalities in both incidence and prevalence of T2DM and the contribution of their risk factors to these inequalities. Studying inequalities in the incidence and prevalence of diabetes across Europe may help to identify general patterns that are independent of the characteristics of specific countries. Furthermore, assessing the role of potential mediators of the relationship between SEP and T2DM incidence or prevalence may aid the design of specific strategies aimed at reducing these inequalities. The aim of this study was to analyze SEP-related inequalities in the prevalence and incidence of T2DM in people aged 50 years and over in Europe between 2004 and 2006 and to describe the contribution of body mass index (BMI) and other possible mediators of SEP-related inequalities in T2DM.
MethodsA cross-sectional and longitudinal analysis of a group of individuals was performed on the basis of the results of two phases of the Survey of Health, Ageing and Retirement in Europe (SHARE) in 2004 and 2006. SHARE is a multidisciplinary and trans-national European study that has collected information on health, SEP and family networks of individuals aged ≥50 years. In this study we included men and women aged ≥50 years who resided in 11 European countries (Austria, Belgium, Denmark, France, Germany, Greece, Italy, Spain, Sweden, Switzerland and The Netherlands). These are the countries that participated in 2004 and had a follow up in the second wave (In 2004, the individual response rate ranged from 73.7% in Spain to 93% in Denmark). All countries attempted proper probability sampling. For more information about SHARE-project it is possible to consult its web page, where there is a lot of additional methodological information (www.share-project.org). The final sample size consisted of individuals who were part of the main sample of SHARE-project, aged 50 years or more.
The prevalence of T2DM was calculated among individuals enrolled in the first phase of the survey in 2004 (n=21,323). T2DM status was self-reported, using the same survey questions in 2004 and 2006: (1) “Has a doctor ever told you that you had any of the conditions on this card? Diabetes or high blood sugar”, or (2) “Do you currently take drugs at least once a week for problems mentioned on this card? Drugs for diabetes”. People who reported diagnosis of diabetes before 20 years of age (1.3%) were considered as they did not have T2DM since they are more likely to be affected by type 1 diabetes. Using data collected during the second phase of the survey in 2006, the cumulative Incidence of T2DM was calculated on the basis of individuals who did not have diabetes in 2004 (n=12,811; 39.9% were lost to follow-up).
Information on educational level (as a measure of SEP) was collected in the first phase of the survey and classified according to the International Standard Classification of Education (ISCED-11) (www.unesco.org). Some categories of this classification were collapsed because of the low numbers of individuals in some groups. The final variable was made up of the following categories: ISCED <3 (lower secondary education or less) or ISCED ≥3 (upper secondary education or more).
The following variables were collected as potential mediators of the association between education and T2DM: BMI was calculated using self-reported weight and height, and was analyzed as both a continuous variable and a 4-level categorical variable: (1) underweight (<18.5kg/m2); (2) normal (18.5–24.9kg/m2); (3) overweight (25–29.9kg/m2); and (4) obese (≥30kg/m2). Smoking status was classified as: (1) currently a smoker, (2) never a smoker, and (3) a former smoker. An individual's alcohol consumption was considered excessive if he reported drinking more than two glasses of beer, cider, wine, spirits or cocktails on five or more days a week. Physical inactivity was defined as never or almost never engaging in moderate or vigorous physical activity such as sports, heavy housework, or a job that involves physical labor. We note that although we have considered the risk factors mentioned above as potential mediators of the link between SEP and T2DM risk, some may not be part of the chain of causality between SEP and T2DM and therefore might be better considered as potential confounders.13 Age and country were treated as potential confounding variables.
Data analysisAll analyses were stratified by sex. Crude and age-adjusted (by the direct method, using the entire sample as the standard for each phase) and country-adjusted (to ensure that all the countries contribute in the same magnitude) prevalence and incidence of T2DM were calculated for each educational level. In order to study the association between educational level and the prevalence and incidence of T2DM, a Poisson regression model with robust variance was used to calculate age-adjusted and country-adjusted prevalence ratio (PR) and relative risk (RR), respectively. To explore the effects of BMI (as a continuous quadratic variable), smoking, physical activity and alcohol consumption, we had fitted additional models, introducing each variable individually and then all variables simultaneously. The contribution of each independent variable in the relationship between prevalence or cumulative incidence of T2DM and educational level was quantified by calculating the percentage change in PR or RR of each model before and after introducing each independent variable. Finally, among the individuals without diabetes in phase I, we compared the characteristics of those who were available for follow-up to those who were not.
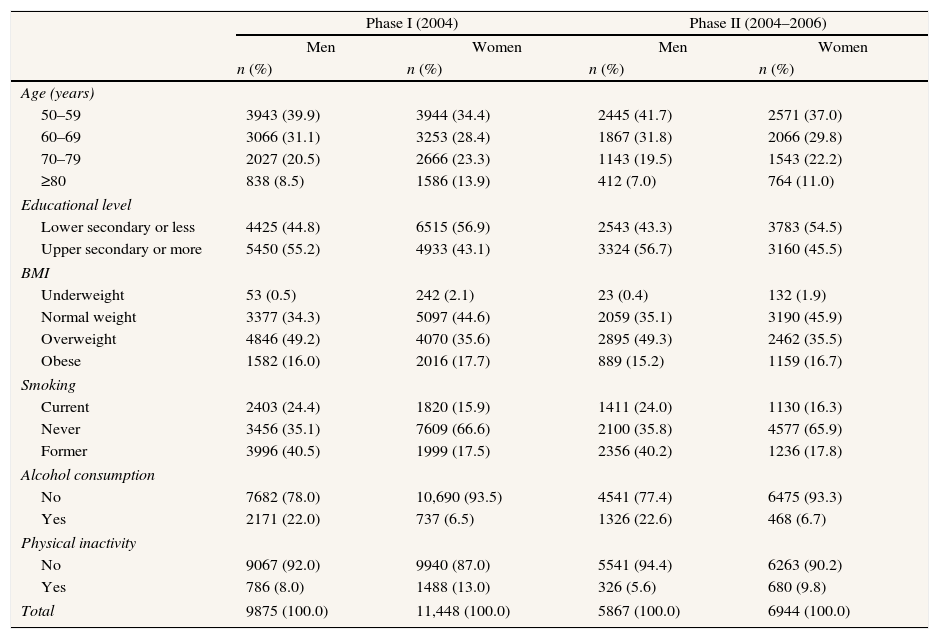
ResultsAmong the participants of phase I, 65% of men and 53% of women were overweight or obese and 92% of men and 87% of women performed some type of physical activity (Table 1). Fifty-five percent of men and 43% of women had upper secondary education or higher. The individuals included in the second phase of the study had similar prevalences of these risk factors, but were generally younger and more physically active than those included in the first phase (both with p<0.01).
Description of study populations.
| Phase I (2004) | Phase II (2004–2006) | |||||||
| Men | Women | Men | Women | |||||
| n (%) | n (%) | n (%) | n (%) | |||||
| Age (years) | ||||||||
| 50–59 | 3943 (39.9) | 3944 (34.4) | 2445 (41.7) | 2571 (37.0) | ||||
| 60–69 | 3066 (31.1) | 3253 (28.4) | 1867 (31.8) | 2066 (29.8) | ||||
| 70–79 | 2027 (20.5) | 2666 (23.3) | 1143 (19.5) | 1543 (22.2) | ||||
| ≥80 | 838 (8.5) | 1586 (13.9) | 412 (7.0) | 764 (11.0) | ||||
| Educational level | ||||||||
| Lower secondary or less | 4425 (44.8) | 6515 (56.9) | 2543 (43.3) | 3783 (54.5) | ||||
| Upper secondary or more | 5450 (55.2) | 4933 (43.1) | 3324 (56.7) | 3160 (45.5) | ||||
| BMI | ||||||||
| Underweight | 53 (0.5) | 242 (2.1) | 23 (0.4) | 132 (1.9) | ||||
| Normal weight | 3377 (34.3) | 5097 (44.6) | 2059 (35.1) | 3190 (45.9) | ||||
| Overweight | 4846 (49.2) | 4070 (35.6) | 2895 (49.3) | 2462 (35.5) | ||||
| Obese | 1582 (16.0) | 2016 (17.7) | 889 (15.2) | 1159 (16.7) | ||||
| Smoking | ||||||||
| Current | 2403 (24.4) | 1820 (15.9) | 1411 (24.0) | 1130 (16.3) | ||||
| Never | 3456 (35.1) | 7609 (66.6) | 2100 (35.8) | 4577 (65.9) | ||||
| Former | 3996 (40.5) | 1999 (17.5) | 2356 (40.2) | 1236 (17.8) | ||||
| Alcohol consumption | ||||||||
| No | 7682 (78.0) | 10,690 (93.5) | 4541 (77.4) | 6475 (93.3) | ||||
| Yes | 2171 (22.0) | 737 (6.5) | 1326 (22.6) | 468 (6.7) | ||||
| Physical inactivity | ||||||||
| No | 9067 (92.0) | 9940 (87.0) | 5541 (94.4) | 6263 (90.2) | ||||
| Yes | 786 (8.0) | 1488 (13.0) | 326 (5.6) | 680 (9.8) | ||||
| Total | 9875 (100.0) | 11,448 (100.0) | 5867 (100.0) | 6944 (100.0) | ||||
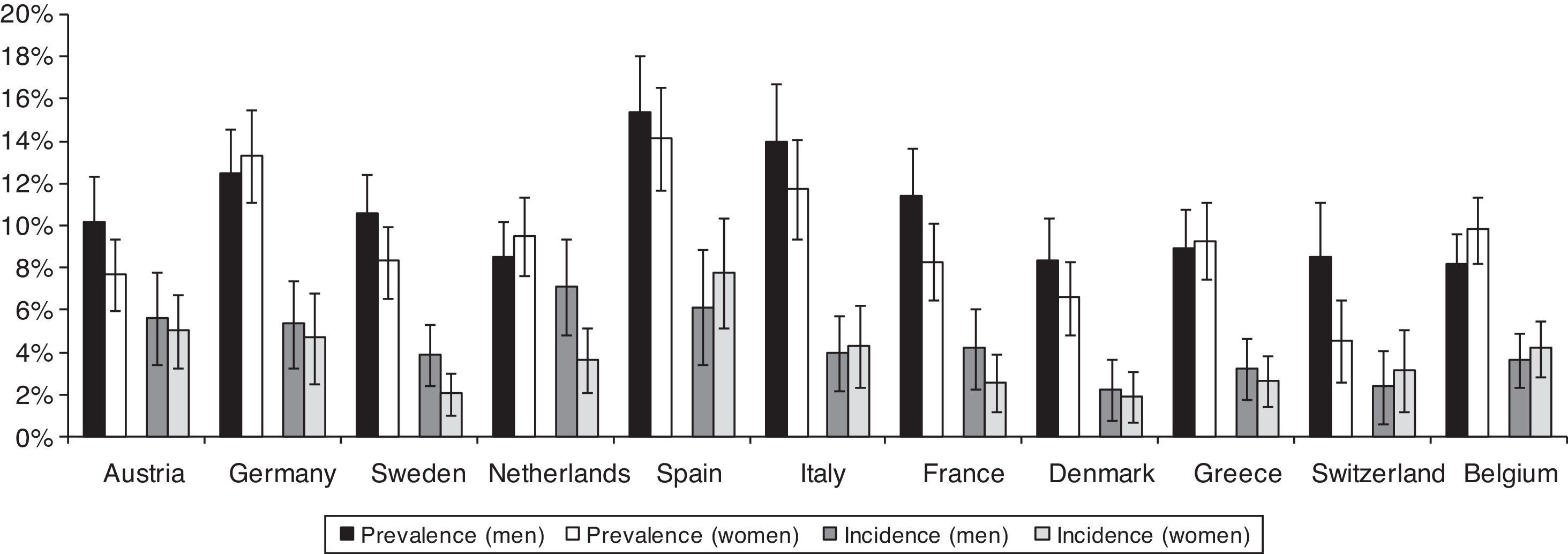
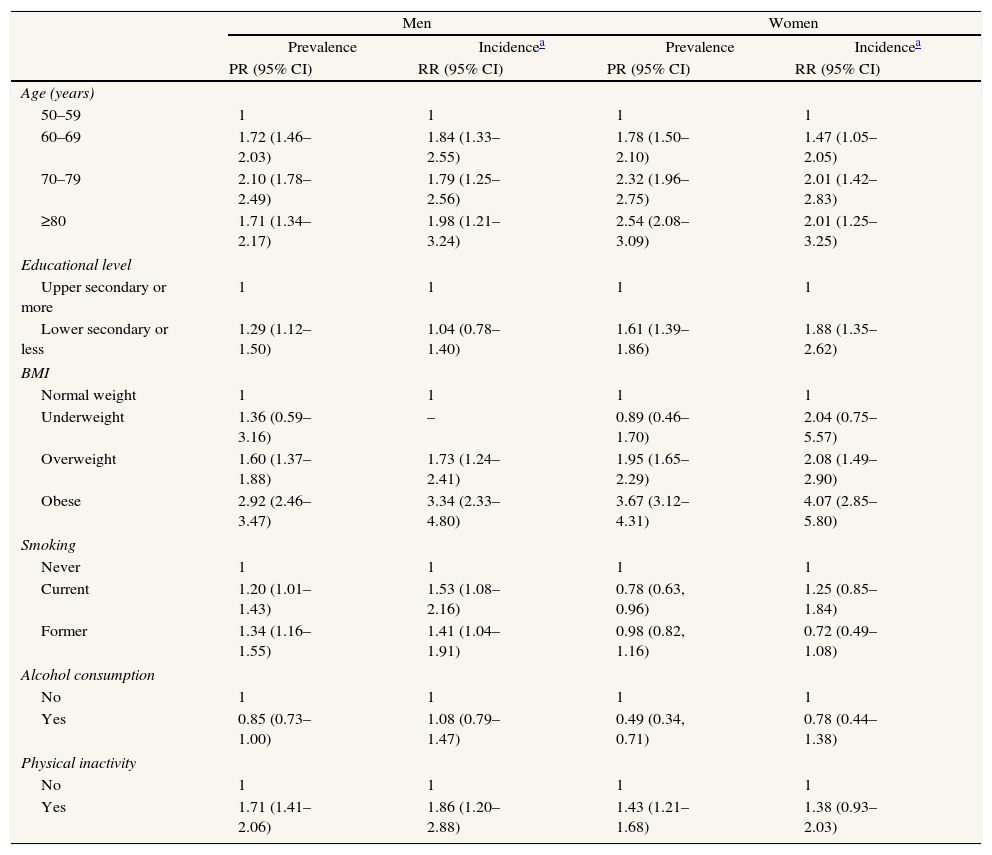
Prevalence of T2DM in men ranges from 8.4% [95% CI: 6.3–10.4] in Denmark to 15.3% [95% CI: 12.5–18.1] in Spain, while in women ranges from 4.5% [95% CI: 2.6–6.5] in Switzerland to 14.2% [95% CI: 11.8–16.6] in Spain. The two-year cumulative incidence of T2DM in men goes from 2.2% [95% CI: 0.8–3.7] in Denmark to 7.1% [95% CI: 4.9–9.4] in The Netherlands, and in women goes from 1.9% [95% CI: 0.7–3.1] in Denmark to 7.8% [95% CI: 5.1–10.4] in Spain (Fig. 1). The age- and country-adjusted prevalence [95% CI] of T2DM in the first phase was 10.2% [95% CI: 9.5–10.8] in men and 8.5% [95% CI: 7.9–9.1] in women. The age- and country-adjusted cumulative incidence of T2DM in the period 2004–2006 was 3.8% [95% CI: 3.3–4.3] in men and 3.4% [95% CI: 2.9–3.9] in women (not shown in the tables). BMI was the strongest predictor of the prevalence (PR among obese versus normal weight 2.92 [95% CI: 2.46–3.47] in men and 3.67 [95% CI: 3.12–4.31] in women) and incidence (RR among obese versus normal weight 3.34 [95% CI: 2.33–4.80] in men and 4.07 [95% CI: 2.85–5.80] in women) of T2DM (Table 2).
Age- and country-adjusted prevalence ratio and relative risk of T2DM in Europeans aged 50 years and over 2004–2006.
| Men | Women | |||||||
| Prevalence | Incidencea | Prevalence | Incidencea | |||||
| PR (95% CI) | RR (95% CI) | PR (95% CI) | RR (95% CI) | |||||
| Age (years) | ||||||||
| 50–59 | 1 | 1 | 1 | 1 | ||||
| 60–69 | 1.72 (1.46–2.03) | 1.84 (1.33–2.55) | 1.78 (1.50–2.10) | 1.47 (1.05–2.05) | ||||
| 70–79 | 2.10 (1.78–2.49) | 1.79 (1.25–2.56) | 2.32 (1.96–2.75) | 2.01 (1.42–2.83) | ||||
| ≥80 | 1.71 (1.34–2.17) | 1.98 (1.21–3.24) | 2.54 (2.08–3.09) | 2.01 (1.25–3.25) | ||||
| Educational level | ||||||||
| Upper secondary or more | 1 | 1 | 1 | 1 | ||||
| Lower secondary or less | 1.29 (1.12–1.50) | 1.04 (0.78–1.40) | 1.61 (1.39–1.86) | 1.88 (1.35–2.62) | ||||
| BMI | ||||||||
| Normal weight | 1 | 1 | 1 | 1 | ||||
| Underweight | 1.36 (0.59–3.16) | – | 0.89 (0.46–1.70) | 2.04 (0.75–5.57) | ||||
| Overweight | 1.60 (1.37–1.88) | 1.73 (1.24–2.41) | 1.95 (1.65–2.29) | 2.08 (1.49–2.90) | ||||
| Obese | 2.92 (2.46–3.47) | 3.34 (2.33–4.80) | 3.67 (3.12–4.31) | 4.07 (2.85–5.80) | ||||
| Smoking | ||||||||
| Never | 1 | 1 | 1 | 1 | ||||
| Current | 1.20 (1.01–1.43) | 1.53 (1.08–2.16) | 0.78 (0.63, 0.96) | 1.25 (0.85–1.84) | ||||
| Former | 1.34 (1.16–1.55) | 1.41 (1.04–1.91) | 0.98 (0.82, 1.16) | 0.72 (0.49–1.08) | ||||
| Alcohol consumption | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.85 (0.73–1.00) | 1.08 (0.79–1.47) | 0.49 (0.34, 0.71) | 0.78 (0.44–1.38) | ||||
| Physical inactivity | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.71 (1.41–2.06) | 1.86 (1.20–2.88) | 1.43 (1.21–1.68) | 1.38 (0.93–2.03) | ||||
PR: prevalence ratio; RR: relative risk; 95%CI: 95% confidence interval; BMI: body mass index.
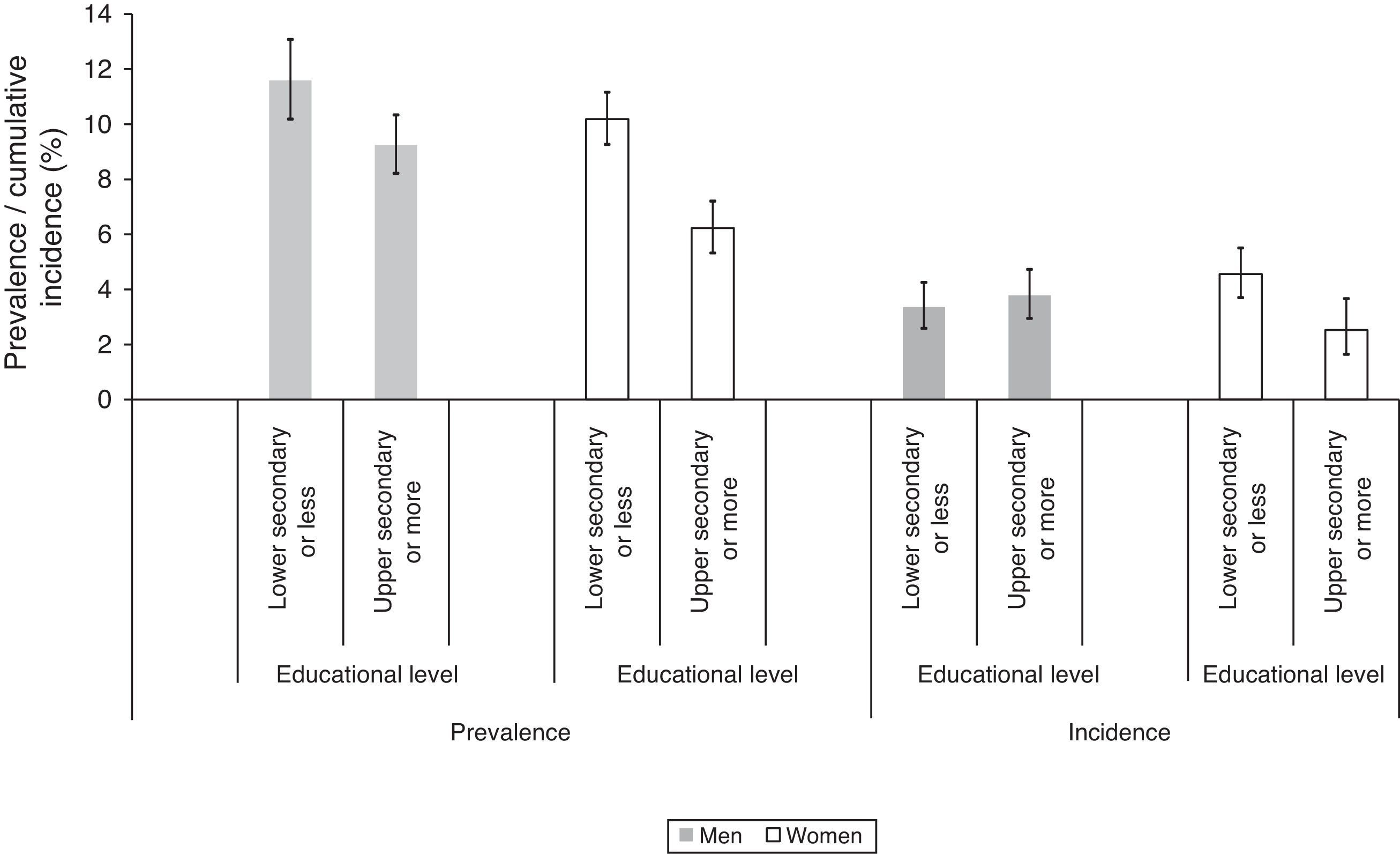
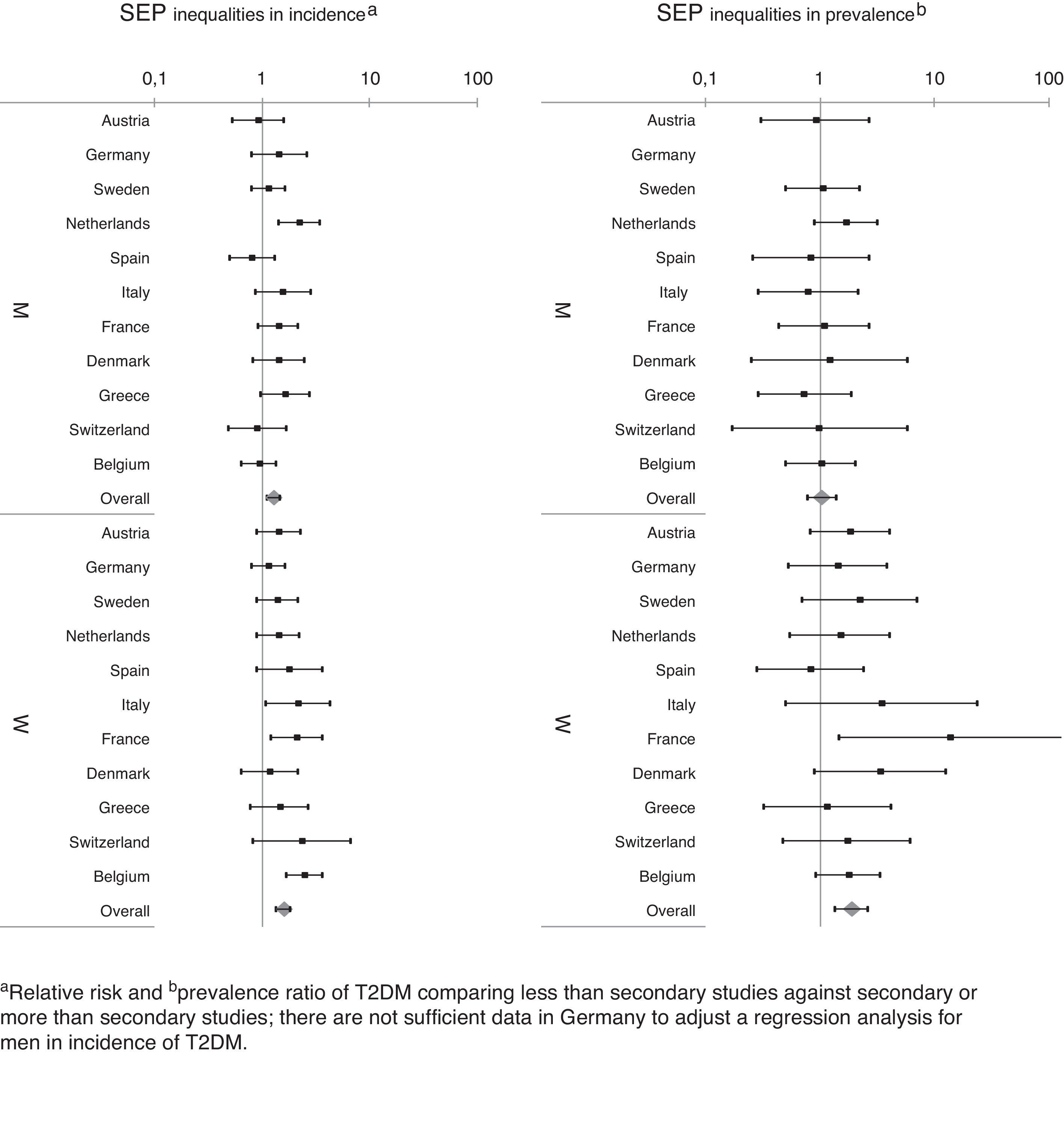
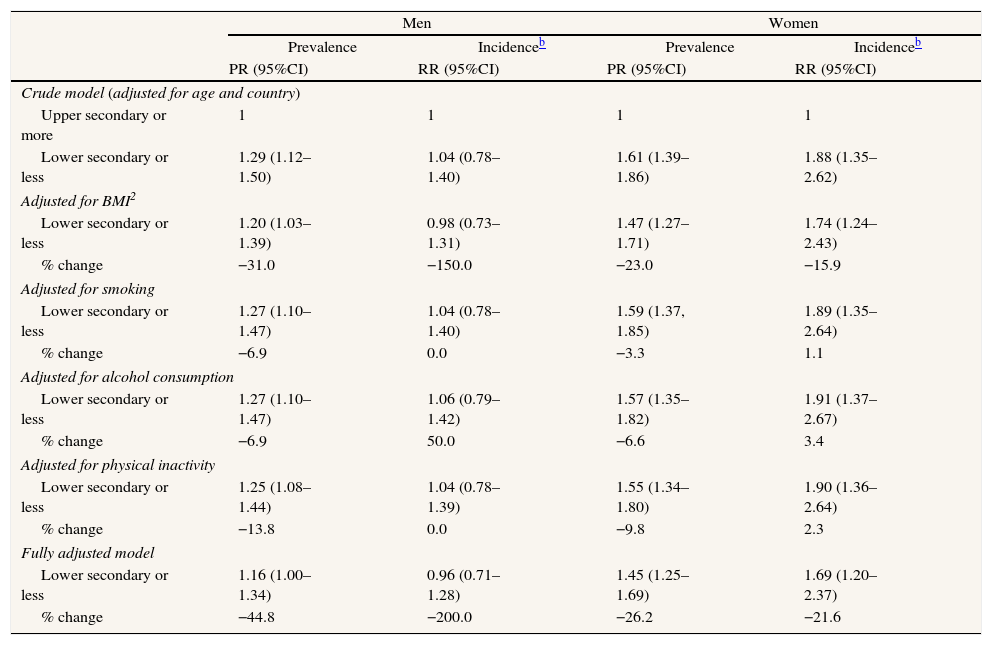
In relation to SEP-related inequalities (Fig. 2 and Table 3), women with a lower secondary level education or less had a higher prevalence of T2DM (PR=1.61 [95% CI: 1.39–1.86]) in the first phase of the survey and a higher incidence (RR=1.88 [95% CI: 1.35–2.62]) during follow-up, compared to women with a upper secondary or more. A similar pattern of SEP-related inequality in the prevalence (PR=1.29 [95% CI: 1.12–1.50]), but not the incidence (RR=1.04 [95% CI: 0.78–1.40]) of T2DM was observed among men. The SEP inequalities in incidence and prevalence in each country are shown in Fig. 3. Overall SEP inequalities in the incidence and prevalence of T2DM were consistent across countries (Fig. 3). In both prevalence and incidence of T2DM, SEP inequalities seemed to be higher in women than in men. For example, point estimates of SEP inequalities in T2DM incidence among men were very close to 1 in each country, while among women they were higher than 1 in the majority of countries. For overall estimations, SEP-related inequalities in the prevalence and incidence of T2DM were significantly greater in women compared to men (p-value for the interaction between sex and SEP: 0.017 for prevalence; 0.005 for incidence).
SEP-related inequalities in the prevalence and incidence of T2DM according to educational level and % changea after adjustment for potential mediator variables. Europeans aged 50 years and over, 2004–2006.
| Men | Women | |||||||
| Prevalence | Incidenceb | Prevalence | Incidenceb | |||||
| PR (95%CI) | RR (95%CI) | PR (95%CI) | RR (95%CI) | |||||
| Crude model (adjusted for age and country) | ||||||||
| Upper secondary or more | 1 | 1 | 1 | 1 | ||||
| Lower secondary or less | 1.29 (1.12–1.50) | 1.04 (0.78–1.40) | 1.61 (1.39–1.86) | 1.88 (1.35–2.62) | ||||
| Adjusted for BMI2 | ||||||||
| Lower secondary or less | 1.20 (1.03–1.39) | 0.98 (0.73–1.31) | 1.47 (1.27–1.71) | 1.74 (1.24–2.43) | ||||
| % change | −31.0 | −150.0 | −23.0 | −15.9 | ||||
| Adjusted for smoking | ||||||||
| Lower secondary or less | 1.27 (1.10–1.47) | 1.04 (0.78–1.40) | 1.59 (1.37, 1.85) | 1.89 (1.35–2.64) | ||||
| % change | −6.9 | 0.0 | −3.3 | 1.1 | ||||
| Adjusted for alcohol consumption | ||||||||
| Lower secondary or less | 1.27 (1.10–1.47) | 1.06 (0.79–1.42) | 1.57 (1.35–1.82) | 1.91 (1.37–2.67) | ||||
| % change | −6.9 | 50.0 | −6.6 | 3.4 | ||||
| Adjusted for physical inactivity | ||||||||
| Lower secondary or less | 1.25 (1.08–1.44) | 1.04 (0.78–1.39) | 1.55 (1.34–1.80) | 1.90 (1.36–2.64) | ||||
| % change | −13.8 | 0.0 | −9.8 | 2.3 | ||||
| Fully adjusted model | ||||||||
| Lower secondary or less | 1.16 (1.00–1.34) | 0.96 (0.71–1.28) | 1.45 (1.25–1.69) | 1.69 (1.20–2.37) | ||||
| % change | −44.8 | −200.0 | −26.2 | −21.6 | ||||
PR: prevalence ratio; RR: relative risk; 95%CI: 95% confidence interval; BMI: body mass index.
The most important mediator risk factor for SEP-related inequalities in both the prevalence and incidence of T2DM was BMI, accounting for 23.0% and 15.9% of the inequality in prevalence and incidence among women, respectively (Table 3). After adjusting for all the potential mediators simultaneously, SEP-related inequalities in the prevalence of T2DM were reduced by 44.8% and 26.2% in men and women, respectively, while inequalities in incidence (that was found statistically significant only among women) were reduced by 21.6% in women. In men, SEP inequalities in incidence of T2DM were not found.
DiscussionIn this study, which tries to ensure a large and representative sample of the European population over 50 years of age, we identified SEP-related inequalities in the prevalence of T2DM in men and women and in the two-year incidence of T2DM among women. BMI appeared as the main mediator variable explaining these inequalities.
LimitationsThe study was based on health survey data and it is generally recognized that respondents report only diagnosed T2DM, which represents just 30–50% of all cases.17,18 However, educational level does not seem to be associated with undiagnosed T2DM, so self-reported diabetes may not be subject to significant reporting bias, and may therefore be a valid tool for evaluating SEP-related inequalities in diabetes prevalence.19
The first phase of this study was carried out using a large representative sample of the European population aged ≥50 years. The second phase, however, whose aim was to evaluate the incidence of T2DM, lasted only 2 years, which may be too short an interval to provide sufficient power to detect real SEP-related inequalities. Therefore, it is possible that statistically significant SEP-related inequalities would be evident in both men and women after a longer follow-up period. The small sample size has not allowed us to obtain conclusive results by country and for this reason a pooled analysis, taking into account the variability of each country, was used for the multivariate analysis. Moreover, we were not able to make conclusions for many different social categories separately, due to the limited incidence cases in the period 2004–2006.
Finally, the proportion of individuals who were lost to follow-up was considerable (39.9%), and we observed some notable differences in the characteristics of these individuals and compared to those of the individuals who were retained. Compared to individuals who were retained in the second phase of the study, men and women who were lost to follow up were older, less obese, were less physically active and with lower educational level (see Table I in Appendix online). In order to evaluate the comparability of each phase of the study, we analyzed SEP-related inequalities in prevalence in the second phase (not shown in any table), and found the PR to be very similar to that in first phase (men: 1.29 and 1.28 [95% CI: 1.09–1.439] in the first and second phase, respectively; women: 1.61 and 1.72 [95% CI: 1.45–2.04] in the first and second phases, respectively). This suggests that losses to follow-up are unlikely to have caused important biases in our results, although incidence results should be taken carefully.
SEP-related inequalities in the prevalence and incidence of T2DMThe socioeconomic inequalities in the prevalence of T2DM observed in our study have been previously described in a representative sample of the younger European population (30–64 years).20 Our study indicates that this pattern of SEP-related inequalities persists in later decades in the European population, and suggests a similar tendency for the incidence of T2DM, consistent with the results of a recent meta-analysis of studies from 6 European countries.8 On the contrary, we observed SEP-related inequalities in the incidence of T2DM among women but not among men. In a study based on English older population,11 these inequalities were found in men and women when household wealth or subjective social status was used as a SEP indicator. However, these inequalities were not found in men when educational level was used,11 which highlights the complexity of SEP indicators and the different aspects that they measure.21 The finding of SEP-related inequalities in the prevalence of T2DM in both genders, but corresponding inequalities in incidence only among women is unexpected, and we can suggest several explanations. SEP-related inequalities in the incidence of T2DM may occur at younger ages in men than in women. In the Whitehall II study, SEP-related inequalities in the incidence of T2DM after 10 years of follow up were found among men aged 33–55 years but not among women.14 Moreover, SEP-related inequalities in total mortality in Europe are stronger among younger men than older men, but do not vary with age among women.22 This could affect the incidence of SEP-related inequalities in T2DM, since men in the lowest SEP have higher risk of both T2DM and mortality (being mortality a competing risk of incidence of T2DM). At any rate, further studies on SEP-related inequalities in the incidence of T2DM with longer follow up and among different age groups and genders are required.
Evaluation of variables that may explain observed inequalities in incidence and prevalence of T2DMVarious factors that could potentially explain the SEP-related inequalities in incidence and prevalence of T2DM observed in this study have been discussed previously.7,11,12,23 In agreement with the majority of previous studies,7,9,24 we found that BMI was an important element of this relationship. It should be noted that detrimental lifestyle choices (smoking, alcohol consumption, physical inactivity and poor diet), especially among individuals with low SEP, could be a way to cope with adverse and stressful situations related to the precariousness of their working and living conditions. These unhealthy behaviors must be understood in the context of limited resources and limited access to health care. In agreement with previous reports,7,11,24,25 the potential mediator variables analyzed in our study explain only a part of these SEP-related inequalities. Some part of the remaining variability may be accounted for misclassification biases such as self-reported BMI or physical activity, or not measuring variables repeatedly over time.12 In addition, unmeasured factors such as family history of diabetes,23,26 diet,24 access to health services,27 labor conditions and other psychosocial factors,14,28 life-course variables10,29 or other socioeconomic variables may also be relevant.11 For example, in a Swedish study characterized by more precise measurements of potential mediator variables (such as physical activity and BMI) and psychosocial factors, the authors were able to explain all SEP-related inequalities in the prevalence of T2DM in women.26
Our study contributes to the ongoing debate on the risk factors that mediate the relationship between SEP-related inequalities and risk of T2DM, indicating that in Europe these inequalities are partly but not entirely explained by various intermediate factors, particularly BMI. Since BMI is a causal mediator of the relationship between SEP and T2DM, strategies to prevent SEP-related inequalities in obesity could partially reduce SEP-related inequalities in T2DM. In order to reduce these inequalities, obesity prevention strategies should focus on contextual conditions that may have greater impact on disadvantaged groups.30 To this end, the WHO has proposed interventions to reduce weight gain and the prevalence of obesity among vulnerable groups in the WHO European region (e.g., interventions in nutrition, physical activity and sedentarism, and in economic and psychosocial factors), and recommends that these interventions be implemented by settings, sectors and actors.31
Gender-related differencesAs in other studies,7 we found more pronounced SEP-related inequalities in T2DM among women than men, and this is thought to be driven by the burden of risk factors, particularly BMI, which is particularly prevalent among women with lower SEP.32,33 However, the gender differences in SEP-related inequalities observed in our study were not entirely explained by BMI or other factors. Psychosocial factors28,34 and the different roles and power relationships between men and women, which were not measured in our study, may partly explain SEP-related inequalities in women.26 Notably, countries such as Finland, Sweden, Denmark and Norway which have a higher percentage of women in the labor market and more redistribute policies than Spain or Italy35 have fewer gender differences in SEP-related inequalities in T2DM.20
ConclusionsIn this study we observed significant SEP-related inequalities in the prevalence of T2DM in a representative sample of European men and women aged 50 years or over. Similar inequalities in the incidence of T2DM were observed in women but not men. These inequalities are partially mediated by BMI and other factors. Future studies should explore structural and psychosocial factors and dietary behavior in order to understand why SEP-related inequalities in diabetes persist in Europe.
Socioeconomic inequalities (SEP) in the prevalence of T2DM are present throughout Europe. The magnitude of SEP Inequalities in incidence of T2DM in Europe population is not known. There are not conclusive results about the residual SEP-related inequalities in the incidence and prevalence of T2DM after adjusting for traditional risk factors.
What does this study add to the literature?There are SEP-related inequalities in the prevalence of T2DM in men and women aged 50 years or more. There are SEP-related inequalities in the incidence of T2DM in people aged 50 years or more only among women. BMI appeared as the main mediator variable explaining the inequalities in incidence and prevalence of T2DM.
A. Espelt, C. Borrell, L. Palència, L. Font-Ribera and A. Kunst contributed to the conception and design of the study. L. Palència contributed to data management. C. Borrell, A. Kunst, R. Gnavi, T. Spadea, L. Palència, L. Font-Ribera, A. Goday and A. Espelt discussed, interpreted and contributed to the results interpretation. A. Espelt wrote the first draft of the paper, which was revised with contributions by all authors.
FundingNone.
Conflict of interestsOne of the authors (C.B.) belongs to the Gaceta Sanitaria editorial committee, but was not involved in the editorial process of the manuscript.
This paper uses data from SHARE release 2.3.0, November 13th 2009. SHARE data collection in 2004–2007 was primarily funded by the European Commission through its 5th and 6th framework programs (project numbers QLK6-CT-2001-00360; RII-CT-2006-062193; CIT5-CT-2005-028857). Additional funding by the US National Institute on Aging (grant numbers U01 AG09740-13S2; P01 AG005842; P01 AG08291; P30 AG12815; Y1-AG-4553-01; OGHA 04-064; R21 AG025169) as well as by various national sources is gratefully acknowledged (see http://www.share-project.org for a full list of funding institutions). This article forms part of the doctoral dissertation of Albert Espelt at the Universitat Pompeu Fabra of Barcelona.